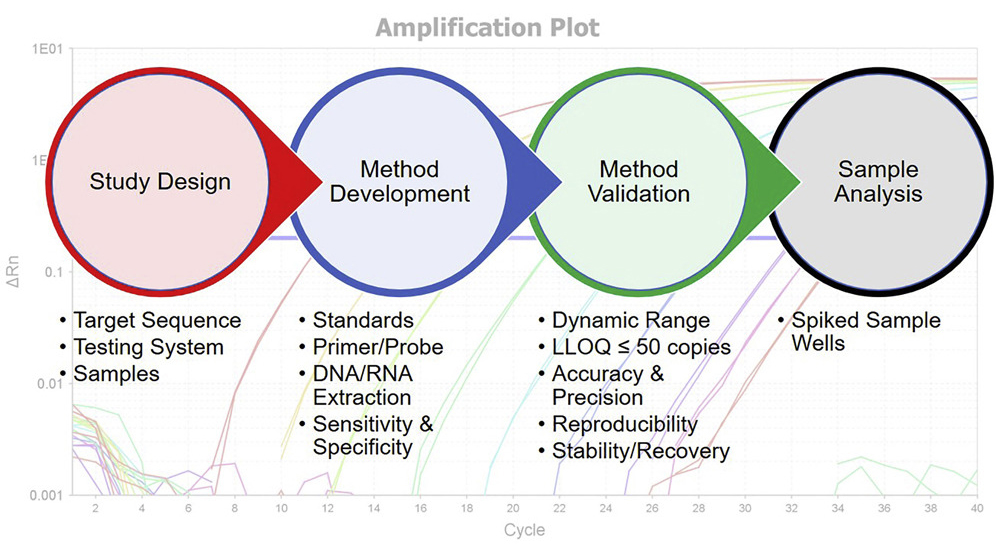
Gene and cell therapy fields have experienced remarkable growth during the past decade. Demands for preclinical and clinical safety assessments of these cell and gene therapy test articles (TAs) have effectively increased the necessity for regulated biodistribution, vector shedding, gene expression, and/or pharmacokinetics bioanalysis studies. This white paper is intended to provide regulatory points to consider when conducting biodistribution and vector shedding studies.
Home » Resources » Blog » White Papers » qPCR and qRT-PCR analysis: Regulatory Points to Consider When Conducting Biodistribution and Vector Shedding Studies
NORTHERN BIO BLOG: WHITE PAPERS
qPCR and qRT-PCR analysis: Regulatory Points to Consider When Conducting Biodistribution and Vector Shedding Studies
Authors
Haiyan Ma, Kristin N. Bell, Rossi N. Loker