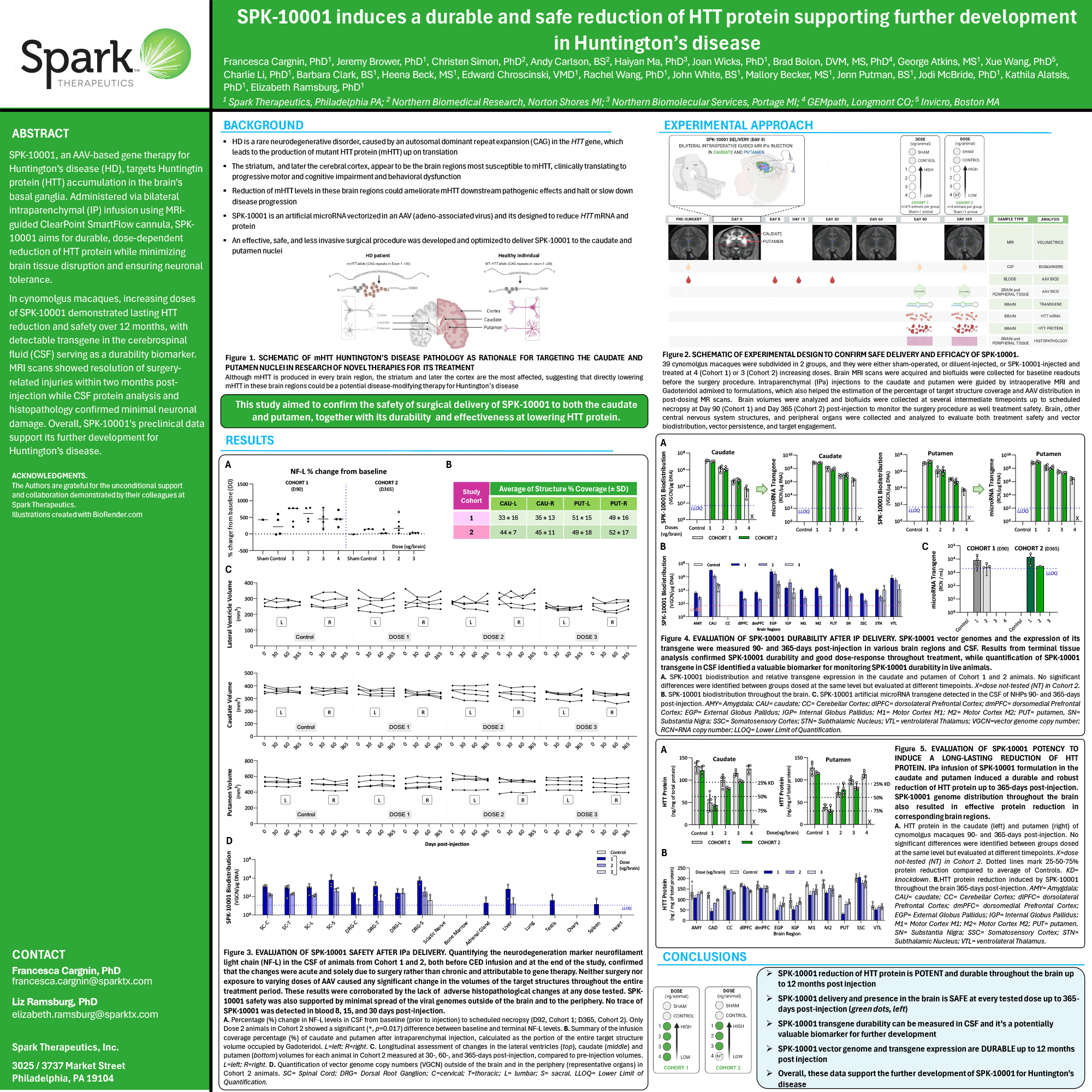
SPK-10001, an AAV-based gene therapy for Huntington’s disease (HD), targets Huntingtin protein (HTT) accumulation in the brain’s basal ganglia. Administered via bilateral intraparenchymal (IP) infusion using MRIguided ClearPoint SmartFlow cannula, SPK-10001 aims for a durable, dose-dependent reduction of HTT protein while minimizing brain tissue disruption and ensuring neuronal tolerance.
In cynomolgus macaques, increasing doses of SPK-10001 demonstrated lasting HTT reduction and safety over 12 months, with detectable transgene in the cerebrospinal fluid (CSF) serving as a durability biomarker. MRI scans showed resolution of surgery-related injuries within two months post-injection, while CSF protein analysis and histopathology confirmed minimal neuronal damage. Overall, SPK-10001’s preclinical data support its further development for Huntington’s disease.
SPK-10001 Induces a Durable and Safe Reduction of HTT Protein Supporting Further Development in Huntington’s Disease
Authors
Francesca Cargnin, Jeremy Brower, Christen Simon, Andy Carlson, Haiyan Ma, Joan Wicks, Brad Bolon, George Atkins, Xue Wang, Charlie Li, Barbara Clark, Heena Beck, Edward Chroscinski, Rachel Wang, John White, Mallory Becker, Jenn Putman, Jodi McBride, Kathila Alatsis, Elizabeth Ramsburg